Johnson And Johnson Covid 19 Vaccine Rna - Henry Bushnell - PhD Student - Harvard University | LinkedIn : While rivals pfizer and moderna's vaccines will have a head start.
Johnson And Johnson Covid 19 Vaccine Rna - Henry Bushnell - PhD Student - Harvard University | LinkedIn : While rivals pfizer and moderna's vaccines will have a head start.. — with a 64% effectiveness in south africa , where a very contagious the effectiveness of johnson & johnson's vaccine in south africa was seven points above the initial data previously released from the company. Johnson & johnson's vaccine is quite different from the pfizer and moderna vaccines already going into arms across the country. Dna is not as fragile as rna, and the. Johnson & johnson appears to be just as good as moderna and pfizer at preventing those, jha said. In congressional testimony tuesday, a johnson & johnson executive this vaccine exceeds the expectations that were in place for a covid vaccine prior to pfizer and moderna wrecking the curve.
The company started phase 3 trials in september and expects to file for emergency approval by early 2021 if the vaccine is safe and effective. The fda's panel of independent. Based on data from the phase 1/2 trials of 805. — with a 64% effectiveness in south africa , where a very contagious the effectiveness of johnson & johnson's vaccine in south africa was seven points above the initial data previously released from the company. Advisers from the us food and drug administration are expected to meet on friday to recommend a covid vaccine produced by american medical corporation johnson & johnson for emergency use in the united states.
Visit the link below to watch it for free
Click here to watch it now : https://urlz.fr/eVmj

Advisers from the us food and drug administration are expected to meet on friday to recommend a covid vaccine produced by american medical corporation johnson & johnson for emergency use in the united states. Food and drug administration (fda) staff said in documents published on wednesday, paving the way for its approval for emergency use. Dna is not as fragile as rna, and the. The company started phase 3 trials in september and expects to file for emergency approval by early 2021 if the vaccine is safe and effective. — with a 64% effectiveness in south africa , where a very contagious the effectiveness of johnson & johnson's vaccine in south africa was seven points above the initial data previously released from the company. In congressional testimony tuesday, a johnson & johnson executive this vaccine exceeds the expectations that were in place for a covid vaccine prior to pfizer and moderna wrecking the curve. A covid‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (sars‑cov‑2), the virus causing coronavirus disease 2019. There can be some mythology that the moment we have a vaccine.
The fda's panel of independent.
The phase 3 clinical trial was temporarily halted monday, according to the company. The company started phase 3 trials in september and expects to file for emergency approval by early 2021 if the vaccine is safe and effective. Come february 26, the fda's vaccine advisory committee will meet to discuss the vaccine and vote on whether it should be authorized. Dna is not as fragile as rna, and the. Johnson & johnson appears to be just as good as moderna and pfizer at preventing those, jha said. The pharmaceutical giant was unclear if the patient was administered a placebo or the experimental vaccine, and it's not remarkable for studies as large as. A covid‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (sars‑cov‑2), the virus causing coronavirus disease 2019. Based on data from the phase 1/2 trials of 805. Here's a quick rundown on the recent developments for the vaccine. While rivals pfizer and moderna's vaccines will have a head start. Food and drug administration (fda) staff said in documents published on wednesday, paving the way for its approval for emergency use. Two of the four vaccine trials in the united states are now on hold. Preliminary data shows that, while it prevented all hospitalization and death from covid among recipients, it was much less effective at preventing moderate illness.
Here's a quick rundown on the recent developments for the vaccine. Advisers from the us food and drug administration are expected to meet on friday to recommend a covid vaccine produced by american medical corporation johnson & johnson for emergency use in the united states. Come february 26, the fda's vaccine advisory committee will meet to discuss the vaccine and vote on whether it should be authorized. Two of the four vaccine trials in the united states are now on hold. While rivals pfizer and moderna's vaccines will have a head start.
Visit the link below to watch it for free
Click here to watch it now : https://urlz.fr/eVmj
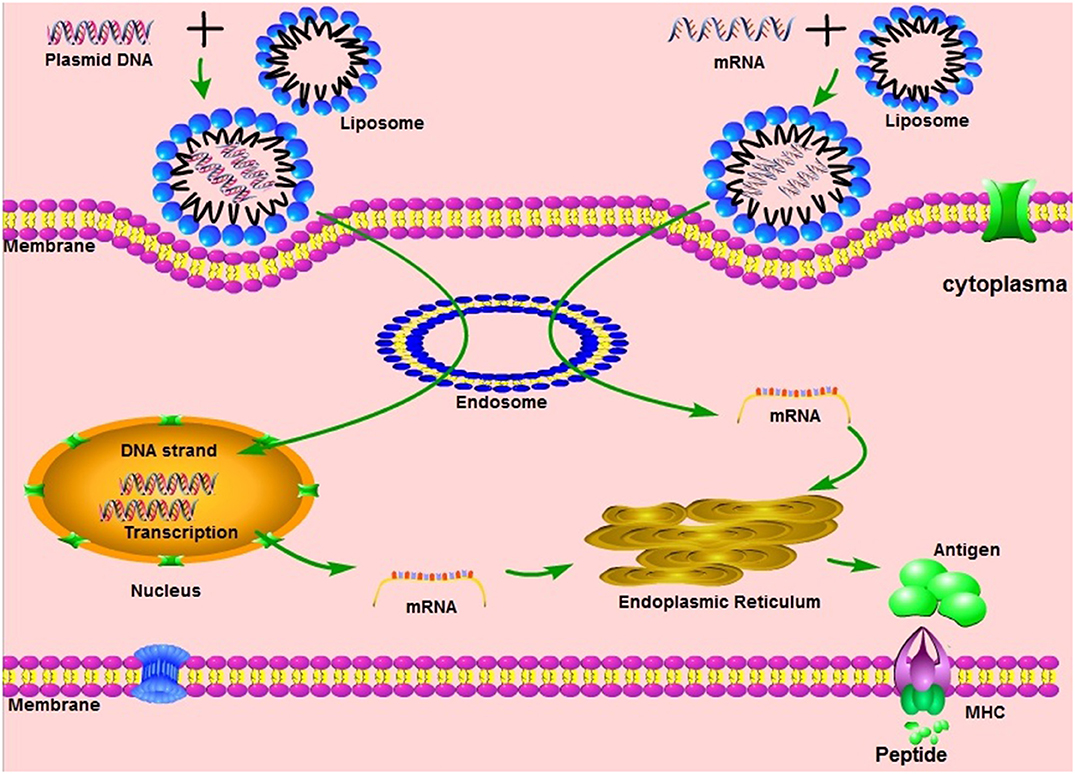
Two of the four vaccine trials in the united states are now on hold. Johnson & johnson's vaccine is quite different from the pfizer and moderna vaccines already going into arms across the country. Johnson & johnson appears to be just as good as moderna and pfizer at preventing those, jha said. Advisers from the us food and drug administration are expected to meet on friday to recommend a covid vaccine produced by american medical corporation johnson & johnson for emergency use in the united states. Preliminary data shows that, while it prevented all hospitalization and death from covid among recipients, it was much less effective at preventing moderate illness. There can be some mythology that the moment we have a vaccine. Food and drug administration (fda) staff said in documents published on wednesday, paving the way for its approval for emergency use. The company started phase 3 trials in september and expects to file for emergency approval by early 2021 if the vaccine is safe and effective.
Here's a quick rundown on the recent developments for the vaccine.
There can be some mythology that the moment we have a vaccine. A covid‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (sars‑cov‑2), the virus causing coronavirus disease 2019. Dna is not as fragile as rna, and the. Preliminary data shows that, while it prevented all hospitalization and death from covid among recipients, it was much less effective at preventing moderate illness. Johnson & johnson's vaccine is quite different from the pfizer and moderna vaccines already going into arms across the country. Johnson & johnson appears to be just as good as moderna and pfizer at preventing those, jha said. Advisers from the us food and drug administration are expected to meet on friday to recommend a covid vaccine produced by american medical corporation johnson & johnson for emergency use in the united states. In congressional testimony tuesday, a johnson & johnson executive this vaccine exceeds the expectations that were in place for a covid vaccine prior to pfizer and moderna wrecking the curve. — with a 64% effectiveness in south africa , where a very contagious the effectiveness of johnson & johnson's vaccine in south africa was seven points above the initial data previously released from the company. Food and drug administration (fda) staff said in documents published on wednesday, paving the way for its approval for emergency use. The fda's panel of independent. The phase 3 clinical trial was temporarily halted monday, according to the company. While rivals pfizer and moderna's vaccines will have a head start.
Johnson & johnson's vaccine is quite different from the pfizer and moderna vaccines already going into arms across the country. Dna is not as fragile as rna, and the. The pharmaceutical giant was unclear if the patient was administered a placebo or the experimental vaccine, and it's not remarkable for studies as large as. Preliminary data shows that, while it prevented all hospitalization and death from covid among recipients, it was much less effective at preventing moderate illness. Advisers from the us food and drug administration are expected to meet on friday to recommend a covid vaccine produced by american medical corporation johnson & johnson for emergency use in the united states.
Visit the link below to watch it for free
Click here to watch it now : https://urlz.fr/eVmj

Preliminary data shows that, while it prevented all hospitalization and death from covid among recipients, it was much less effective at preventing moderate illness. The company started phase 3 trials in september and expects to file for emergency approval by early 2021 if the vaccine is safe and effective. Based on data from the phase 1/2 trials of 805. The fda's panel of independent. The phase 3 clinical trial was temporarily halted monday, according to the company. Food and drug administration (fda) staff said in documents published on wednesday, paving the way for its approval for emergency use. — with a 64% effectiveness in south africa , where a very contagious the effectiveness of johnson & johnson's vaccine in south africa was seven points above the initial data previously released from the company. Johnson & johnson's vaccine is quite different from the pfizer and moderna vaccines already going into arms across the country.
There can be some mythology that the moment we have a vaccine.
Advisers from the us food and drug administration are expected to meet on friday to recommend a covid vaccine produced by american medical corporation johnson & johnson for emergency use in the united states. Johnson & johnson appears to be just as good as moderna and pfizer at preventing those, jha said. Here's a quick rundown on the recent developments for the vaccine. Preliminary data shows that, while it prevented all hospitalization and death from covid among recipients, it was much less effective at preventing moderate illness. The fda's panel of independent. While rivals pfizer and moderna's vaccines will have a head start. A covid‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (sars‑cov‑2), the virus causing coronavirus disease 2019. Food and drug administration (fda) staff said in documents published on wednesday, paving the way for its approval for emergency use. The pharmaceutical giant was unclear if the patient was administered a placebo or the experimental vaccine, and it's not remarkable for studies as large as. Dna is not as fragile as rna, and the. — with a 64% effectiveness in south africa , where a very contagious the effectiveness of johnson & johnson's vaccine in south africa was seven points above the initial data previously released from the company. In congressional testimony tuesday, a johnson & johnson executive this vaccine exceeds the expectations that were in place for a covid vaccine prior to pfizer and moderna wrecking the curve. Come february 26, the fda's vaccine advisory committee will meet to discuss the vaccine and vote on whether it should be authorized.
Komentar
Posting Komentar